 Ghonaym Biotech and Engineering
Ghonaym Biotech and Engineering of Houston
 Ghonaym Biotech and Engineering
Ghonaym Biotech and Engineering Quality work begins with quality knowledge. In the pharmaceutical and regulated industries, expertise isn’t optional—it’s the foundation of everything we do. Our commitment to understanding the science, compliance standards, and operational nuances behind every project ensures precision, reliability, and excellence from concept to completion.


High-performance air handling, balancing, and environmental stability for compliant spaces.

Process piping, utilities, and equipment integration with full GMP documentation.
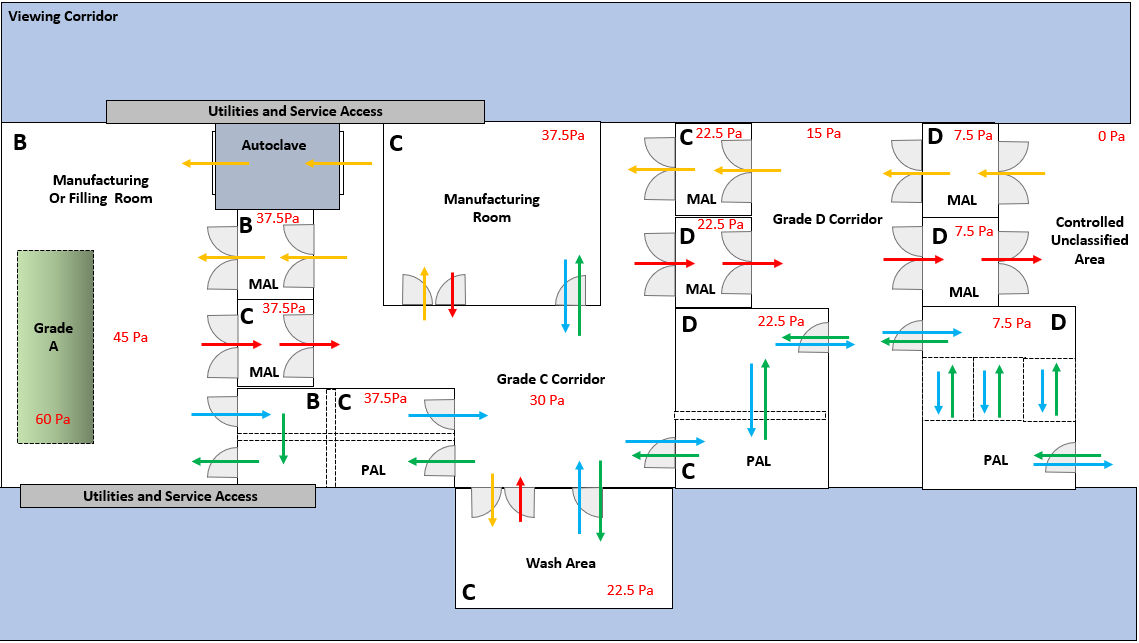
ISO 7/8 suites, commissioning, qualification, and validation with EM programs.